Hydrometallurgy in the Processing of REE
Once a REE-containing concentrate has been produced by physical ore beneficiation the next steps are dissolution, separation and purification of rare earth elements. For this, hydrometallurgy is one of the main procedures. Hydrometallurgical treatment is well developed for some of the commonly processed rare earth minerals such as monazite (Kumari et al., 2015) but more work was needed to optimise such techniques for the key minerals for EURARE.
Three unit operations have to be considered in hydrometallurgy:
- Dissolution of the rare earth content in acid, sometimes at elevated pressure and temperature.
- Separation of the different REE into pure and concentrated solutions, by solvent extraction or ionic liquid extraction and ion exchange.
- Generation of individual and pure rare earth elements.
There are many alternative ways to carry out a complete metal recovery process, and to develop such a process it is necessary to combine different operations in a unique way.
Different dissolution and separation methods were studied by EURARE partners including MEAB, RWTH, NTUA and KUL.
Solvent Extraction
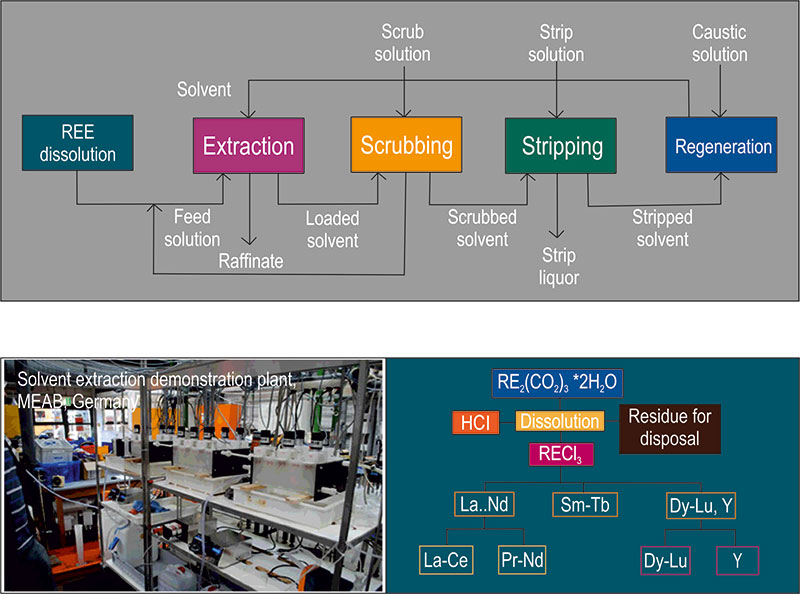
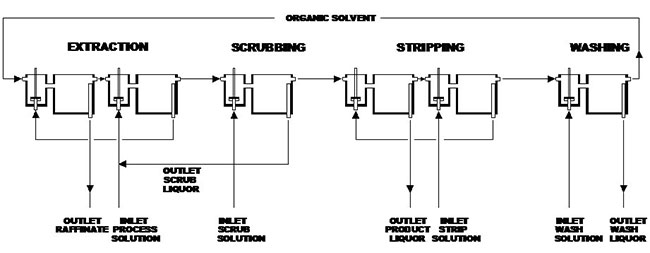
Solvent extraction (SX) in hydrometallurgy is an important process for separating and obtaining the REE. Solvent extraction is a selective separation procedure for isolating and concentrating substances from aqueous solutions with the aid of an immiscible organic solvent. The procedure has rapidly gained industrial importance in hydrometallurgy and has been widely adopted for the recovery and separation of the different REE into pure and concentrated solutions.
In a solvent extraction procedure, an aqueous feed solution containing REE is mixed with an organic solvent containing an organic reagent. The REE react with the reagent to form compounds that are more soluble in the organic solvent and, consequently, are extracted into the solvent.
In order to clean the organic solvent from co-extracted substances, a scrubbing function may be included. The REE are subsequently re-extracted (stripped) from the organic solvent by mixing it with a "new" aqueous solution in which the REE are again more soluble. The REE are concentrated into this solution, at concentrations often 10–100 times that of the original feed solution, achieved by adjustment of the flow rates.
Various processes for separation and purification of rare earth elements, in groups or individually, utilize the small differences in basicity resulting from decrease in the ionic radius from the LREE to the HREE. The basicity differences influence the solubility of the salts, the hydrolysis of ions, and the formation of complex species. These properties also form the basis of the separation techniques.
At MEAB, where the researchers worked with REE carbonate produced from hydrometallurgical studies, the separation of heavy (HREE: dysprosium-lutetium (Dy–Lu), Y), medium (MREE: samarium-terbium (Sm–Tb)) and light (LREE: La–Nd) rare earth elements was achieved. Y was separated from the HREE fraction and a mixture of praseodymium (Pr) and Nd was separated from the LREE fraction. Finally, Y was successfully separated from the LREE fraction.
General Separation Procedures for the REE



In addition to a tri-valent oxidation state, cerium, praseodymium and terbium can also occur in the tetra-valent state. Europium, samarium and ytterbium exhibit a di-valent state. Selective oxidation and reduction of these rare earth elements is useful in an effective separation procedure because in the di-valent and tetra-valent states the rare earth elements have a different chemical and physical behaviour compared to that in the tri-valent state.
Organo-phosphorus acids are typical cation exchange reagents used in separation of the REE, and involve the displacement of a hydrogen ion from the reagents by the extracted rare earth element. The distribution coefficient in an organo-phosphorus rare earth system increases with an increase in rare earth atomic number, allowing separation to be achieved. The separation of co-extracted rare earth elements can be enhanced by the use of a scrubbing function. The exchange reaction is also pH dependent.
In addition to the organo-phosphorus acids, solvation reagents were studied and used for REE separations. Tri butyl phosphate (TBP) appears to be very effective under certain conditions.
In an operation using an organo-phosphorus acid or a solvating reagent, yttrium is anomalous, acting as a HREE in chloride media, and as a LREE in thiocyanate media. This behaviour was utilized in a proposed process for preparing high purity yttrium oxide.
Of the various other basic organic reagents, only quaternary ammonium salts, marketed as Aliquat 336, turned out to be promising for separation and purification of REE.
The EURARE project included laboratory batch investigations to evaluate the solvent extraction process conditions, which was used to prepare pilot plant activities, and for verification and determination of equipment and investment costs.
Information provided by EURARE partner MEAB
Key references
Kumari, A, Panda, R, Jha, M K, Kumar, J R, Lee, J Y. 2015. Process development to recover rare earth metals from monazite mineral: A review. Minerals Engineering 79, 102–115.